Background: Chemotherapy and its combination are the mainstay of lymphoma and multiple myeloma (MM) treatment, with high-intensity regimens frequently causing cancer therapy-induced thrombocytopenia(CTIT), which results in dose reductions and treatment delays. Hetrombopag, a novel oral non-peptide thrombopoietin receptor agonist, is approved in China for immune thrombocytopenic purpura and severe aplastic anemia. This study retrospectively evaluated the efficacy of hetrombopag in treating CTIT among lymphoma and MM patients.
Methods: In this retrospective study, lymphoma and MM patients experiencing≥grade 2 thrombocytopenia (PLT<75×10 9/L) after anti-tumor treatment at the Fifth Medical Center of PLA General Hospital from July 2021 to May 2022 who received hetrombopag were included. All patients were treated with 5 mg hetrombopag once daily until PLT≥100×10 9/L or treatment discontinuation as directed by physicians.
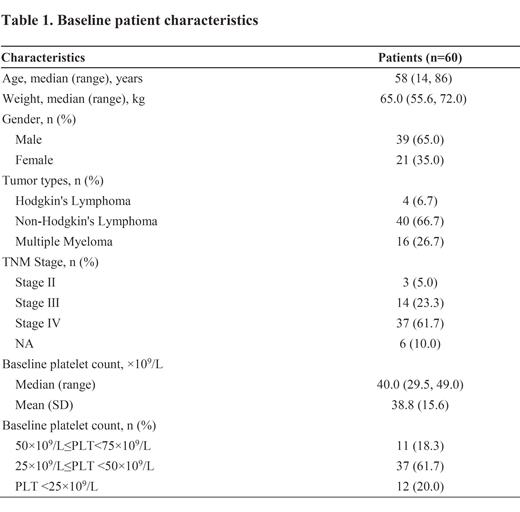
Results: A total of 60 patients (4 Hodgkin's lymphoma, 40 non-Hodgkin's lymphoma, and 16 MM) were analyzed. Their baseline characteristics are presented in Table 1. The median age was 58.0 years (range 14, 86), with 51 (85%) stage Ⅲ/Ⅳdiseases. Most patients received chemotherapy alone (40.0%, 24/60) or combined with targeted therapy (48.3%, 29/60). Notably, 90% patients received a combination of ≥3 chemotherapy drugs. The mean PLT before hetrombopag treatment was (38.8±15.6) ×10 9/L, with 81.7% (49/60) of grade 3/4 thrombocytopenia (PLT<50×10 9/L). The median treatment duration with hetrombopag was 8 days (range 2, 28). The median time for PLT recovery to 75×10 9/L, and 100×10 9/L from hetrombopag initiation was 7 days (range 3, 14), and 9 days (range 3, 13), respectively. The mean PLT counts on days 3, 5, and 10 of hetrombopag treatment showed notable increases to (40.9±30.9) ×10 9/L, (54.9±37.8) ×10 9/L, and (94.4±70.5) ×10 9/L, respectively. Only 3 patients (5%) developed bleeding events, and 50% of patients avoided platelet transfusion. No hetrombopag-related adverse events were observed.
Conclusion: With the limit of a retrospective evaluation, we observed that hetrombopag might increase PLT, shorten the duration of thrombocytopenia, with no significant toxicity, for lymphoma and MM patients who developed CTIT of ≥grade 2 in this study.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Hetrombopag is a novel oral non-peptide thrombopoietin receptor agonist approved in China for immune thrombocytopenic purpura and severe aplastic anemia. In clinical practice, physicians have tried to use Hetrombopag to raise platelets in patients who develop thrombocytopenia during antitumor therapy. This study retrospectively evaluated the efficacy of hetrombopag in treating anti-cancer therapy-induced thrombocytopenia among lymphoma and multiple myeloma patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal